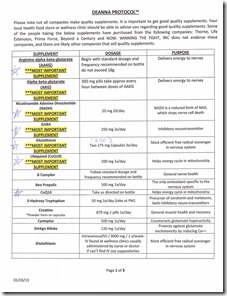
Here’s some information I’m looking at and researching… and testing
I’m in my second week (as of 1/28/2013), but am ramping doses slowly to avoid stomach or bowel issues. There’s a lot of high dose anti-oxidants in this regimen..
Here’s the welcome letter from the creator… http://www.winningthefight.net/
Here’s what I started with the 1st 2 weeks ($300 later for a calculated 60 day trial)….
- Glycine 500mg 1x/day
- AAKG 7g 1x/day
- NADH 20g 1x/day
- GABA 250mg 1x/day
- Glutathione 250mg 1x/day
- Ubiquinol 200mg 1x/day
- CoQ-10 200 mg 1x/day
- Methyl B-12 complex 5000mcg 1x/day
(partial listing…. for now)
items already being taken:
- Clonazopam 1.5mg night
- Baclofen 10mg 2x/day
- Zoloft 50mg
- Robinol 1mg
next level to be started 2/4/2013 (changes in red)
- Glycine 500mg 2x/day
- AAKG 10g 1x/day
- NADH 20g 2x/day
- GABA 250mg 2x/day
- Glutathione 500mg 1x/day
- Ubiquinol 200mg 1x/day
- CoQ-10 400mg 1x/day
- Methyl B-12 complex 5000mcg 1x/day